top of page
2015 – 2018

60. Silver-Catalyzed Regio- and Stereoselective Formal Carbene Insertion into Unstrained C-C sigma-Bonds of 1,3-Dicarbonyls
Z. Liu, X. Zhang, M. Virelli, G. Zanoni, E. A. Anderson and X. Bi
iScience, 2018, 8, 54-60
doi: 10.1016/j.isci.2018.09.006

59. Selectivity in Transition Metal-catalyzed Cyclizations: Insights from Experiment and Theory
R. S. Paton and E. A. Anderson
Chimia, 2018, 72, 614-620
doi: 10.2533/chimia.2018.614



58. Dual Oxidation State Tandem Catalysis in the Palladium-Catalyzed Isomerization of Alkynyl Epoxides to Furans
C. Arroniz, G. Chaubet and E. A. Anderson
ACS Cat., 2018, 8, 8290–8295
doi: 10.1021/acscatal.8b02248.
57. Ermine Revolt
E. A. Anderson and B. G. Davis
ACS Central Sci., 2018, 4, 781-782
doi: 10.1021/acscentsci.8b00440.
56. Synthesis and Applications of Highly Functionalized 1-Halo-3-Substituted Bicyclo[1.1.1]pentanes
D. F. J. Caputo, C. Arroniz, A. Dürr, J. J. Mousseau, A. F. Stepan, S. J. Mansfield and E. A. Anderson
Chem. Sci., 2018, 9, 5295-5300
doi: 10.1039/C8SC01355A.

55. Catalyst-Dependent Chemoselective Formal Insertion of Diazo Compounds into C–C or C–H Bonds of 1,3-Dicarbonyl Compounds
Z. Liu, P. Sivaguru. G. Zenoni, E. A. Anderson and X. Bi
Angew. Chem. Int. Ed., 2018, 57, 8927-8931
doi: 10.1002/anie.201802834.

54. Advances in the synthesis of nitroxide radicals for use in biomolecule spin labelling
M. M. Haugland, J. E. Lovett, E. A. Anderson
Chem. Soc. Rev., 2017, 47, 668-680
doi: 10.1039/C6CS00550K.

53. α-Substituted vinyl azides: an emerging functionalized alkene
J. Fu, G. Zanoni, E. A. Anderson, X. Bi
Chem. Soc. Rev., 2017, 46, 7208-7228
doi: 10.1039/C7CS00017K.

52. Synthesis and Applications of Yndiamides
S. J. Mansfield, K. E. Christensen, A. L. Thompson, K. Ma, M. W. Jones, A. Mekareeya and E. A. Anderson
Angew. Chem. Int. Ed., 2017, 56, 14428-14432
doi: 10.1002/anie.201706915.

51. Total synthesis of the Schisandracaenortriterpenoid rubriflordilactone A
G. Chaubet, S. S. Goh, M. Mohammad, B. Gockel, M.-C. A. Cordonnier, H. Baars, A. W. Phillips and E. A. Anderson
Chem. Eur. J., 2017, 23, 14080-14089
doi: 10.1002/chem.201703229.

50. Silver-Catalyzed Stereoselective Aminosulfonylation of Alkynes
Y. Ning, Q. Ji, P. Liao, E. A. Anderson, X. Bi
Angew. Chem. Int. Ed., 2017, 56, 13805-13808
doi: 10.1002/anie.201705122.
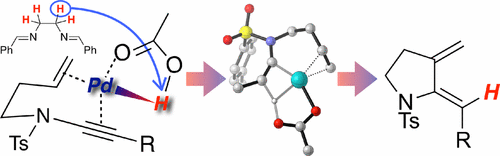
49. Mechanistic Insight into Palladium-Catalyzed Cycloisomerization: A Combined Experimental and Theoretical Study
A. Mekareeya, P. R. Walker, A. Couce-Rios, C. D. Campbell, A. Steven, R. S. Paton and E. A. Anderson
J. Am. Chem. Soc., 2017, 139, 10104-10114
doi: 10.1021/jacs.7b05436.
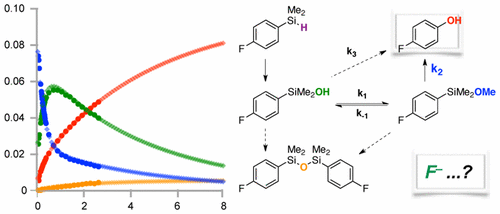
48. Mechanistic Study of Arylsilane Oxidation through 19F NMR Spectroscopy
E. J. Rayment, A. Mekareeya, N. Summerhill and E. A. Anderson
J. Am. Chem. Soc., 2017, 139, 6138-6145
doi: 10.1021/jacs.7b00357.

47. 2'-Alkynylnucleotides: A Sequence- and Spin Label-Flexible Strategy for EPR Spectroscopy in DNA
M. M. Haugland, A. H. El-Sagheer, R. J. Porter, J. Peña, T. Brown, E. A. Anderson, and J. E. Lovett
J. Am. Chem. Soc., 2016, 138, 9069-9072
doi: 10.1021/jacs.6b05421.

46. Cascade polycyclisations in natural product synthesis
R. Ardkhean, D. F. J. Caputo, S. M. Morrow, H. Shi, Y. Xiong and E. A. Anderson
Chem. Soc. Rev., 2016, 45, 1557-1569
doi: 10.1039/C5CS00105F.

45. Computational ligand design in enantio- and diastereoselective ynamide [5+2] cycloisomerization
R. N. Straker, Q. Peng, A. Mekareeya, R. S. Paton and E. A. Anderson.
Nature Commun., 2016, 7, 10109
doi: 10.1038/ncomms10109.

44. Combining cycloisomerization with trienamine catalysis: a regiochemically flexible enantio- and diastereoselective synthesis of hexahydroindoles
V. Chintalapudi, E. A. Galvin, R. L. Greenaway and E. A. Anderson
Chem. Comm., 2016, 52, 693-696
doi: 10.1039/C5CC08886K.

43. Total Synthesis of (+)-Rubriflordilactone A
S. S. Goh, G. Chaubet, B. Gockel, M.-C. A. Cordonnier, H. Baars, A. W. Phillips and E. A. Anderson
Angew. Chem. Int. Ed., 2015, 54, 12618-12621
doi: 10.1002/anie.201506366

42. Ynamide carbopalladation: A flexible route to mono-, bi- and tricyclic azacycles
C. D. Campbell, R. L. Greenaway, O. T. Holton, P. R. Walker, H. A. Chapman, C. A. Russell, G. Carr, A. L. Thomson and E. A. Anderson.
Chem. Eur. J., 2015, 21, 12627-12639
doi: 10.1002/chem.201501710.

41. A robust and modular synthesis of ynamides
Steven J. Mansfield, Craig D. Campbell, Michael W. Jones, and E. A. Anderson
Chem. Comm., 2015, 51, 3316-3319
doi: 10.1039/C4CC07876D.
bottom of page