2019 – 2021
94. Synthesis of α-Quaternary Bicyclo[1.1.1]pentanes through Synergistic Organophotoredox and Hydrogen Atom Transfer Catalysis
J. Nugent, A.J. Sterling, N. Frank, J.J. Mousseau, E.A. Anderson*
Org. Lett. 2021, 23, 8628–8633
doi:10.1021/acs.orglett.1c03346

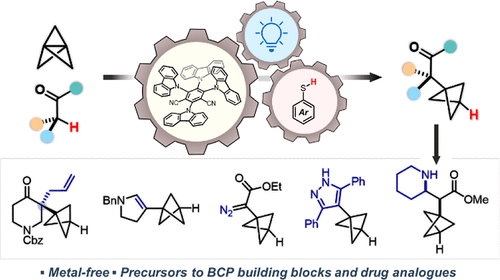
93. Beyond strain release: Delocalisation-enabled organic reactivity
A. Sterling, R. Smith, E. Anderson, F. Duarte
ChemRxiv, 2021
doi:10.26434/chemrxiv-2021-n0xm9

92. Synthesis and Applications of Polysubstituted Bicyclo[1.1.0]butanes
R.E. McNamee, A.L. Thompson, E.A. Anderson*
J. Am. Chem. Soc., 2021, 143, 50, 21246–21251
https://doi.org/10.1021/jacs.1c11244

91. Synthesis of the C1–C27 Fragment of Stambomycin D Validates Modular Polyketide Synthase-Based Stereochemical Assignments
J. Lim, V. Chintalapudi, H.G. Gudmundsson, M. Tran, A. Bernasconi, A. Blanco, L. Song, G.L. Challis, and E.A. Anderson*
Org. Lett., 2021, 23, 19, 7439–7444
doi: https://doi.org/10.1021/acs.orglett.1c02650

90. Regio- and stereoselective addition of Brønsted acids to yndiamides: Synthesis of N,O,N- and N,S,N-trisubstituted ketene acetals
O.L Garry, S.J. Mansfield, and E.A. Anderson*
Synthesis, 2021, 53, 22, 4221-4230
doi: https://doi.org/10.1055/a-1638-5783

89. Synthesis of Polysubstituted Fused Pyrroles by Gold-Catalyzed Cycloisomerization/1,2-Sulfonyl Migration of Yndiamides
P.J. Smith, Y. Jiang, Z. Tong, H.D. Pickford, K.E. Christensen, J. Nugent, and E.A. Anderson*
Org. Lett., 2021, 23, 6547–6552
doi: https://doi.org/10.1021/acs.orglett.1c02360

88. Recent progress in the total synthesis of pyrrole-containing natural products (2011–2020)
N. Singh,‡ S. Singh,‡ S. Kohli, A. Singh, H. Asiki, G. Rathee, R. Chandra,* and E.A. Anderson*
Org. Chem. Front., 2021, 8, 5550-5573
doi: https://doi.org/10.1039/D0QO01574A

87. Twofold Radical-Based Synthesis of N,C-Difunctionalized Bicyclo[1.1.1]pentanes
H. D. Pickford, J. Nugent, B. Owen, J. J. Mousseau, R. C. Smith and
E.A. Anderson*
J. Am. Chem. Soc., 2021, 143, 9729–9736
doi: https://doi.org/10.1021/jacs.1c04180

86. Au(I)-Catalyzed Oxidative Functionalization of Yndiamides
Z. Tong, O. L. Garry, P. J. Smith, Y. Jiang, S. J. Mansfield and E. A. Anderson*
Org. Lett., 2021, 23, 4888–4892
doi: https://doi.org/10.1021/acs.orglett.1c01625

85. Synthesis of 1,3-Disubstituted Bicyclo[1.1.0]butanes via Directed Bridgehead Functionalization
R. McNamee, M. M. Haugland, J. Nugent, R. Chan, K. Christensen and E. A. Anderson*
Chem. Sci., 2021, 12, 7480–7485
doi: https://doi.org/10.1039/D1SC01836A

84. Marine alkaloids as bioactive agents against protozoal neglected tropical diseases and malaria
A. G. Tempone,* P. Pieper, S. E. T. Borborema, F. Thevenard, J. H. G. Lago, S. L. Croft,* E. A. Anderson*
Nat. Prod. Rep., 2021, 38, 2214-2235
doi: https://doi.org/10.1039/D0NP00078G

83. Direct catalytic asymmetric synthesis of α-chiral bicyclo[1.1.1]pentanes
M. L. J. Wong, A. J. Sterling, J. J. Mousseau, F. Duarte,* E. A. Anderson*
Nat. Commun., 2021, 12, 1644
doi: https://doi.org/10.1038/s41467-021-21936-4
Blog post: https://go.nature.com/3uWge10
Highlighted in SYNFORM: 10.1055/s-0040-1720531

82. Advances in polycyclization cascades in natural product synthesis
Y. Jiang, R. E. McNamee, P. J. Smith, A. Sozanschi, Z. Tong,
E. A. Anderson*
Chem. Soc. Rev., 2021, 50, 58–71
doi: https://doi.org/10.1039/D0CS00768D

81. Synthesis and Structure–Activity Relationship of Dehydrodieugenol B Neolignans against Trypanosoma cruzi
C. E. Sear, P. Pieper, M. Amaral, M. M. Romanelli, T. A. Costa-Silva, M. M. Haugland, J. A. Tate, J. H. G. Lago, A. G. Tempone*, E. A. Anderson*
ACS Infect. Dis., 2020, 6, 2872–2878
doi: 10.1021/acsinfecdis.0c00523

80. Site-Selective C–H Benzylation of Alkanes with N-Triftosylhydrazones Leading to Alkyl Aromatics
Z. Liu, S. Cao, W. Yu, J. Wu, F. Yi, E. A. Anderson*, X. Bi*
Chem, 2020, 6, 2110–2124
doi: 10.1016/j.chempr.2020.06.031

79. Copper-Catalyzed Intramolecular C–H Alkoxylation of Diaryltriazoles: Synthesis of Tricyclic Triazole Benzoxazines
X. Ma, H. Li, H. Xin, W. Du, E. A. Anderson*, X. Dong*, Y. Jiang*
Org. Lett., 2020, 22, 5320–5325
doi: 10.1021/acs.orglett.0c01517

78. Structure-activity relationship study of cytotoxic neolignan derivatives using multivariate analysis and computation-aided drug design
F. S. de Sousa, J. L. Baldim, R. A. Azevedo, C. R. Figueiredo, P. Pieper,
C. E. Sear, E. A. Anderson, J. H. G. Lago
Bioorg. Med. Chem. Lett., 2020, 30, 127349
doi: 10.1016/j.bmcl.2020.127349

77. Synthesis of All-carbon Disubstituted Bicyclo[1.1.1]pentanes by Iron-Catalyzed Kumada Cross-Coupling
J. Nugent,‡ B. R. Shire,‡, D. F. J. Caputo, H. D. Pickford, F. Nightingale,
I. T. T. Houlsby, J. J. Mousseau, E. A. Anderson
‡equal author contribution
Article: Angew. Chem. Int. Ed., 2020, 59, 11866–11870
doi: 10.1002/anie.202004090
Featured in: Org. Process Res. Dev., 2020, 24, 8, 1351–1363
doi: 10.1021/acs.oprd.0c00344

76. Rationalizing the diverse reactivity of [1.1.1]propellane through 𝜎-𝜋-delocalization
A. J. Sterling, A. B. Dürr, R. C. Smith, E. A. Anderson, F. Duarte
Chem. Sci., 2020, 11, 4895–4903
doi: 10.1039/D0SC01386B

75. 2′-Alkynyl spin-labelling is a minimally perturbing tool for DNA structural analysis
J. S. Hardwick, M. M. Haugland, A. H. El-Sagheer, D. Ptchelkine,
F. R. Beierlein, A. N. Lane, T. Brown, J. E. Lovett, E. A. Anderson
Nucleic Acids Research, 2020, 48, 2830–2840
doi: 10.1093/nar/gkaa086

74. A Modular, Enantioselective Synthesis of Resolvins D3, E1, and Hybrids
F. Urbitsch, B. L. Elbert, J. Llaveria, P. E. Streatfeild, E. A. Anderson
Org. Lett., 2020, 22, 1510–1515
doi: 10.1021/acs.orglett.0c00089

73. Enantioselective synthesis and anti-parasitic properties of aporphine natural products
P. Pieper, E. McHugh, M. Amaral, A. G.Tempone, E. A.Anderson
Tetrahedron., 2019, 76, 130814
doi: 10.1016/j.tet.2019.130814

72. Synthesis of Cyclic Alkenyl Dimethylsiloxanes from Alkynyl Benzyldimethylsilanes and Application in Polyene Synthesis
H. G. Gudmundsson, C. J. Kuper, D. Cornut, F. Urbitsch, B. L. Elbert,
E. A. Anderson
J. Org. Chem., 2019, 84, 14868-14882
doi: 10.1021/acs.joc.9b01664

71. Convergent Total Syntheses of (–)-Rubriflordilactone B and (–)-pseudo-Rubriflordilactone B
M. Mohammad, V. Chintalapudi, J. M. Carney, S. J. Mansfield, P. Sanderson, K. E. Christensen, E. A. Anderson
Angew. Chem. Int. Ed., 2019, 58, 18177–18181
doi: 10.1002/anie.201908917

70. Dehydrodieugenol B derivatives as antiparasitic agents: Synthesis and biological activity against Trypanosoma cruzi
D. D. Ferreira, F. S. Sousa, T. A. Costa-Silva, J. Q. Reimão, A. C. Torrecilhas, D. M. Johns, C. E. Sear, K. M. Honorio, J. H. G. Lago, E. A. Anderson, A. G. Tempone
Eur. J. Med. Chem., 2019, 176, 162-174
doi: 10.1016/j.ejmech.2019.05.001

69. A semi-synthetic neolignan derivative from dihydrodieugenol B selectively affects the bioenergetic system of Leishmania infantum and inhibits cell division
M. Amaral, F. S. de Sousa, T. A. Costa Silva, A. Jimenez G. Junior, N. N. Taniwaki, D. M. Johns, J. H. G. Lago, E. A. Anderson & A. G. Tempone
Scientific Reports, 2019, 6, 6114
doi: 10.1038/s41598-019-42273-z

68. Silver-Catalyzed C- to N-Center Remote Arene Migration
Y. Ning, A. Mekareeya, K. R. Babu, E. A. Anderson and X. Bi
ACS Catalysis, 2019, 9, 4203-4210
doi: 10.1021/acscatal.9b00500



67. A General Route to Bicyclo[1.1.1]pentanes through Photoredox Catalysis
J. Nugent,* C. Arroniz,* B. R. Shire, A. J. Sterling, H. D. Pickford, M. L. J. Wong, S. J. Mansfield, D. F. J. Caputo, B. Owen, J. J. Mousseau, F. Duarte and E. A. Anderson
*equal author contribution
ACS Catalysis, 2019, 9, 9568-9574
doi: 10.1021/acscatal.9b03190
66. A General Copper-Catalyzed Synthesis of Ynamides from 1,2-Dichloroenamides
S. J. Mansfield, R. C. Smith, J. R. J. Yong, O. L. Garry, and E. A. Anderson
Org. Lett., 2019, 21, 2918-2922
doi: 10.1021/acs.orglett.9b00971.
65. Synthesis of Enantioenriched α-Chiral Bicyclo[1.1.1]pentanes
M. L. J. Wong, J. J. Mousseau, S. J. Mansfield and E. A. Anderson.
Org. Lett., 2019, 21, 2408-2411
doi: 10.1021/acs.orglett.9b00691.

64. Palladium-catalysed ligand-free reductive Heck cycloisomerisation of 1,6-en-α-chloro-enamides
Y. Hou, J. Ma, H. Yang, E. A. Anderson, A. Whiting and N. Wu.
Chem. Commun., 2019, 55, 3733-3736
doi: 10.1039/C9CC00537D.

63. Direct Transformation of Terminal Alkynes into Amidines by a Silver-Catalyzed Four-Component Reaction
B. Liu, Y. Ning, M. Virelli, G. Zanoni, E. A. Anderson and X. Bi.
J. Am. Chem. Soc., 2019, 141, 1593-1598
doi: 10.1021/jacs.8b11039.

62. Use of trifluoroacetaldehyde N-tfsylhydrazone as a trifluorodiazoethane surrogate and its synthetic applications
X. Zhang, Z. Liu, X. Yang, Y. Dong, M. Virelli, G. Zanoni, E. A. Anderson and X. Bi.
Nature Commun., 2019, 10, 284
doi: 10.1038/s41467-018-08253-z.
